Introduction
In a previous post, the phosphorescent properties of several glow-in-the-dark decorative paints were evaluated. The active phosphor in these paints was likely to be europium-doped strontium aluminate. In this post, we examine the emission characteristics of doped strontium aluminate in more detail and record the phosphorescence spectra of these fascinating materials.
Some Technical Background
Phosphorescent materials emit light, particularly in the visible region of the electromagnetic spectrum. This phenomenon has been known since the beginning of the 17th century when in 1602 the Italian shoemaker V. Casciarolo first noticed strong luminescence from the mineral barytes (BaSO4). In Italy during this period barytes was referred to as Bologna stone.
At that time, of course, the chemical and physical mechanism of this luminescent afterglow was not understood. But in the early 20th century, phosphorescent materials (phosphors) based on zinc sulphide (ZnS) that had been doped with copper ions were discovered that had persistent afterglows of several minutes. The addition of cobalt ions to ZnS as a second dopant improved the duration of the afterglow and the materials were further developed with the use of the alkaline earth sulphides calcium sulphide (CaS) and strontium sulphide (SrS).
However, phosphors based on sulphides posed several technical issues that limited their use. They were susceptible to hydrolysis (reaction with moisture) which made them unstable since they then converted to the sulphate form which degraded their luminescence properties. Radioisotopes, such as promethium (Pm-147) and tritium (H-3) were added to the phosphors in attempts to improve matters. Although this did result in increased phosphorescence to some extent, their use was limited due to obvious safety and environmental concerns.
In the late 1960’s, strontium aluminate compounds that had been doped with lanthanide elements, especially europium (Eu2+) ions, attracted the attention of researchers and were found to be highly efficient phosphors. Research for improved luminescent materials continued and in the mid-1990’s Eu-doped strontium aluminates were further improved by Japanese researchers at the Nemoto company. For the first time, they introduced the trivalent rare earth ion dysprosium (Dy3+) into the host aluminate matrix to act as a co-dopant with Eu2+. This was found to increase significantly the duration of luminescence compared to existing materials at that time.
Today, research into extending the duration of luminescence of doped strontium aluminates continues apace. The compounds exhibit some of the longest persistence times for phosphors and are the focus of considerable scientific interest, largely due to being environmentally friendly, of low chemical toxicity, excellent chemical stability, high quantum yield and the broad range of excitation wavelengths that can be employed to produce light emission.
Besides their persistent luminescent properties they have also become of great interest for potential applications in plasma display panels, in fluorescent lamps, night safety signage, improved LED’s and many other areas of research where long duration luminescent properties are required.
Nature of the Phosphorescence
Strontium aluminates occur in several different stoichiometries. The basic host matrix has the molecular formula SrAl2O4, but SrAl4O7, Sr3Al2O6, SrAl12O19 and Sr4Al14O25 all exist and they all have different 3D crystallographic structures.
Although other lanthanide rare earth elements such as trivalent cerium (Ce3+) and the other alkaline earths calcium and barium have been studied, and still are being researched, Eu2+ ions in strontium aluminates, with Dy3+ as a co-dopant, has been the most frequently investigated system. This is because it exhibits the longest phosphorescence lifetime and the Sr2+ and Eu2+ ions have very similar ionic radii (1.21 and 1.20 Å respectively). Consequently, Eu2+ ions can readily replace Sr2+ ions in the host aluminate lattice. This has been confirmed by electron paramagnetic resonance (EPR) measurements (see references).
The electron configuration of a neutral Eu atom, when written out fully, is 1s22s22p63s23p64s23d104p65s24d105p66s24f7!
Now that is quite a mouthful! So it is usually abbreviated to [Xe]6s24f7. [Xe] represents the electron configuration of xenon, where all the electron shells (orbitals) up to and including the 5p shell are completely occupied and filled according to the Aufbau principle. As such, these lower shell electrons do not participate in any energy transitions: they are too strongly held by the nucleus.
The divalent europium ion with two fewer electrons, therefore, has the configuration [Xe]4f7. Note that it is the two electrons in the 6s shell that are removed to form the doubly charged ion Eu2+.
For an explanation of electron configurations and state nomenclature, and the filling of atomic orbitals by electrons, a useful description is found here.
When excited by UV light, a valence electron from Eu2+ is promoted from the 4f7 ground state to the first higher energy 4f65d1 state. (Usually, when describing energy transitions as in this case, the [Xe] term is often omitted for simplicity.) The subsequent de-excitation of energy back down to the 4f7 ground state occurs with the emission of a photon.
Now the interaction of the 4f orbitals of Eu2+ with the surrounding host matrix anions is relatively weak. Electrons residing in the 5d orbitals of Eu2+, on the other hand, are fully exposed to the surrounding ligands and their environment. A consequence of this is that the wavelength of the transition from the excited 4f65d1 state to the 4f7 state is strongly influenced by the chemical and electronic environment of the host matrix surrounding the Eu2+ ion. It is similarly affected by the lattice network and stoichiometry created during the synthesis of the aluminate powders. As a result, emission wavelengths from the divalent Eu2+ ion can shift appreciably across the whole visible spectrum and the colours of the phosphors produced can be controlled and varied – a very useful property.
Sample Preparation
Eu2+/ Dy3+ doped strontium aluminate powders, labelled only as “yellow”, “green” and “blue” were purchased online from the usual source (Amazon of course). No technical information came with the powders other than a statement that they were doped strontium aluminate.
Plain sunlight or artificial room light is sufficient to give a weak afterglow to these powders when observed in the dark, but the phosphorescence is much more pronounced when irradiated with long wavelength UV or a high intensity blue-violet LED lamp, as this short video demonstrates.
A spectrum recording phosphorescent emission from the Eu2+ transition can be obtained directly from the powders using an optical fibre feeding into a spectrometer. However, a more effective way is to formulate a small quantity of each powder with a non-reactive acrylic or epoxy resin. The resin must be of good quality and form a perfectly clear and transparent solid after curing. The cheaper epoxy resins (such as Araldite™) generally become straw coloured after curing and are unsuitable because any colour from the resin will degrade the phosphorescence intensity.
So if you want to try this experiment, spend a little more money on obtaining a high quality two-part resin formulation that is clear and transparent.
5-10 grams of each aluminate powder were thoroughly mixed with 15 ml of one component (the resin) and then an equal volume of the hardener was added and mixed again. Careful mixing is required to minimize creating bubbles in the viscous liquid mixture. If bubbles do form, slight warming of the mixture will decrease the viscosity temporarily before the curing reaction starts and gentle stirring will eliminate them. The resulting mixture was poured into a silicone kitchen mould and left for 24 hours for the polymerization reaction to run to completion and the resin is then fully cured. Various stages in the mould preparation can be seen in this short gallery:
Phosphorescence Spectra
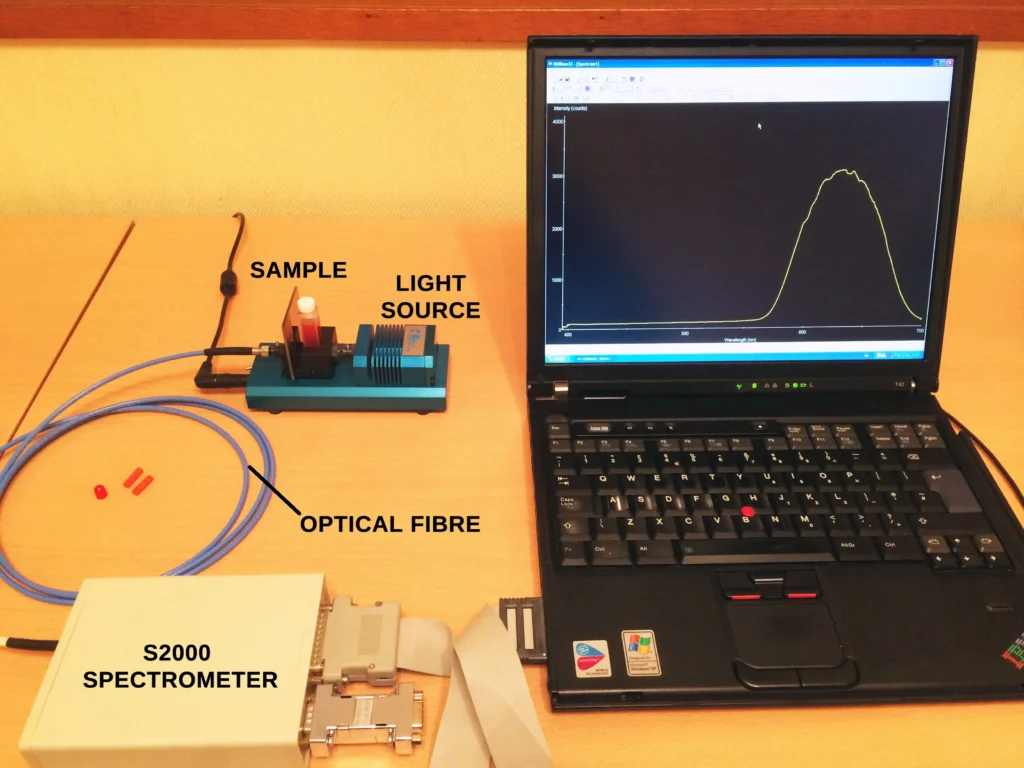
Spectra for each sample were recorded using an optical fibre directed at the surface of the resin which transmitted the light into an Oriel MS125 spectrograph and iDus CCD detector. The grating groove density was 600 lpm and slit width 200 µm..
Spectra for each of the three samples under long UV-violet excitation are given in the following figures with explanations.
Yellow Sample
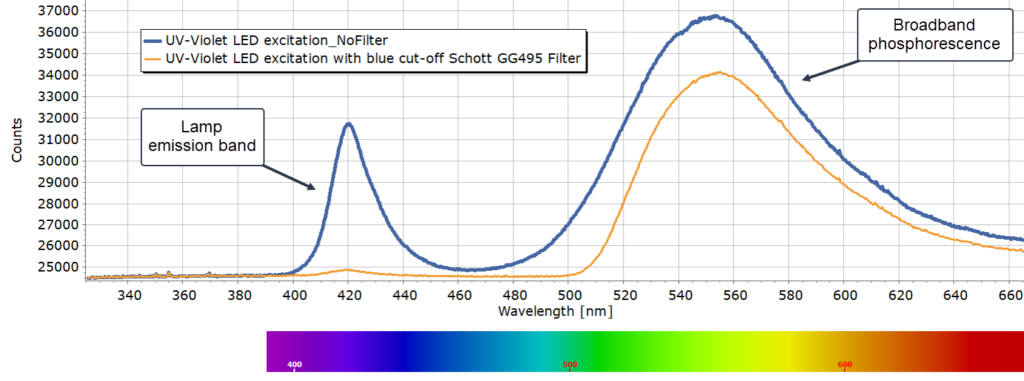
In this first trace, a blue cut-off filter was used to remove scattered light emission from the UV lamp that enters the spectrometer. Use of a yellow GG495 filter removes the vast majority of the excitation source light.
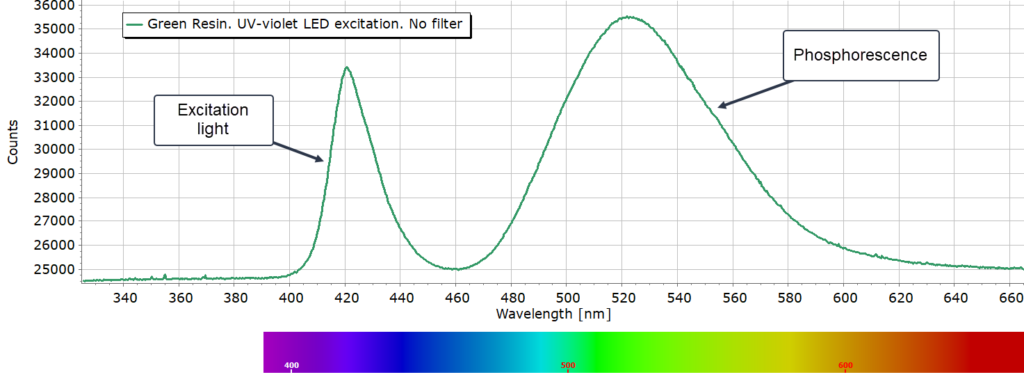
Green Sample
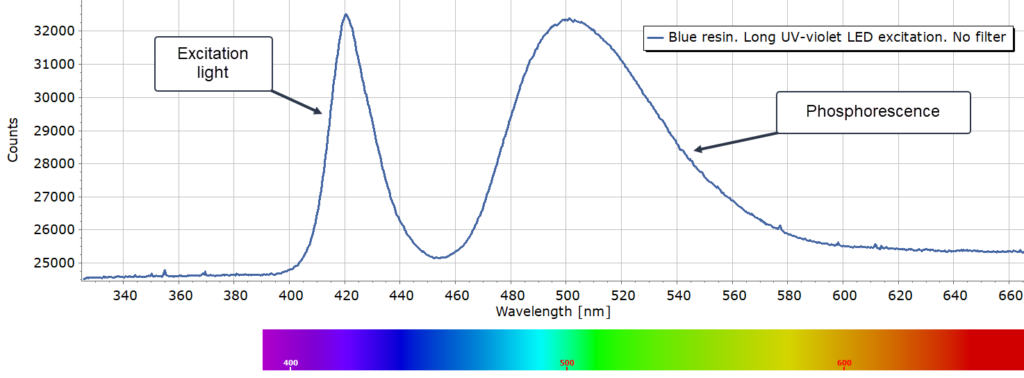
Blue Sample
Phosphorescence Decay and Persistence
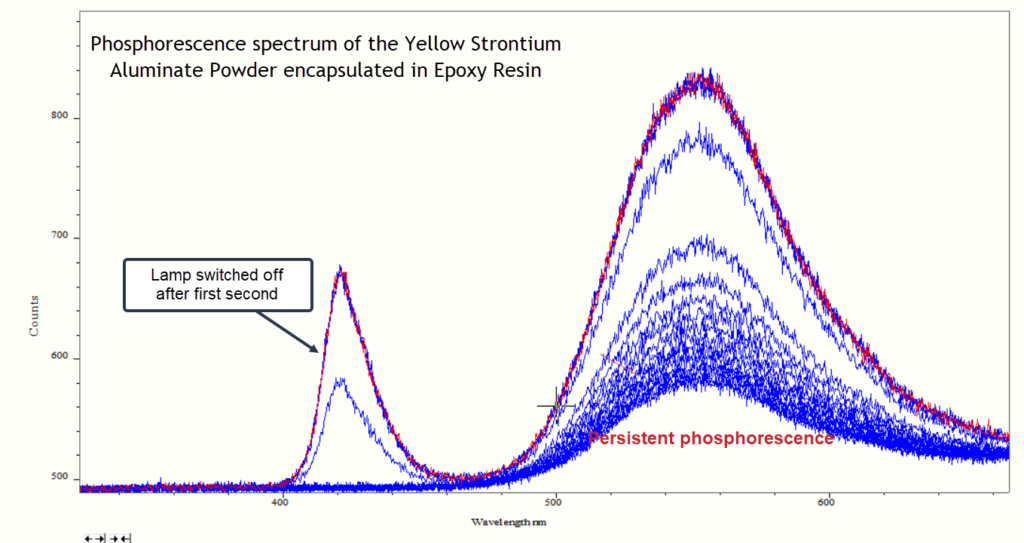
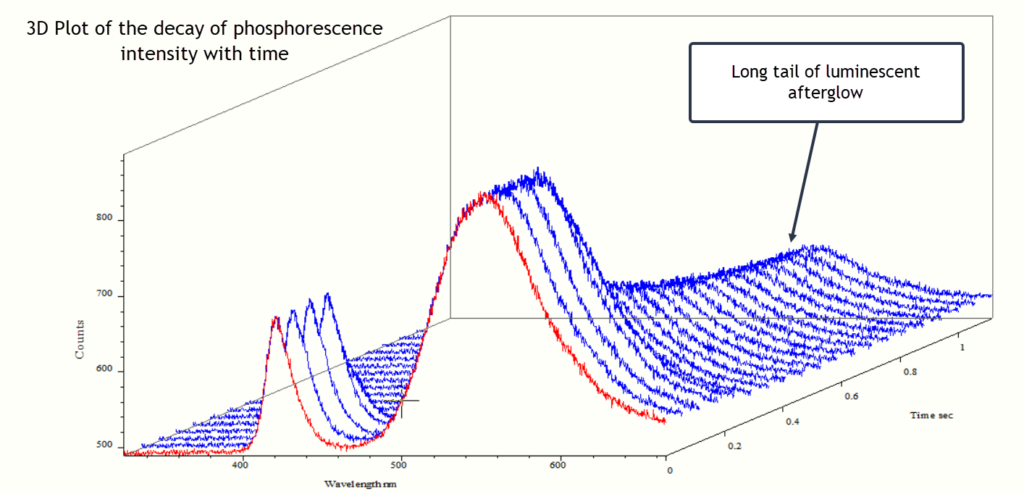
The traces below are multiple spectra of one of the resins (the yellow sample) presented as overlay plots. The emission band around 420 nm is the excitation source and the broad band of phosphorescence has a maximum around 560 nm. The UV lamp was switched off after about 1 second and the phosphorescence signal very rapidly drops but still persists at a lower intensity. It is this persistence of phosphorescence which lasts for several minutes.
The traces below are of the same yellow sample presented as a 3D plot with time in seconds on the z axis.
Finally, this video has condensed over 30 minutes of phosphorescence into a 1 minute clip showing the persistence of luminescence taken in a darkened room.
Conclusions
Extending the duration of phosphorescence from alkaline earth aluminates that have been doped with ions of the lanthanide group elements continues to be a very active field of research. Future improvements are likely to be seen in the general area of persistent luminescent materials for applications in road signage, LED’s, glow-sticks for emergency services and many other fields requiring unpowered lighting.
References
- Matsuzawa et al. A New Long Phosphorescent Phosphor with High Brightness, SrAl2O4:Eu2+, Dy3+, J. Electrochem. Soc. 1996, 143, 2670-2673.
- Dorenbos, P., The Eu3+ charge transfer energy and the relation with the band gap of compounds. Journal of Luminescence 2005,111 (1-2), 89-104.
- Dorenbos, P., Absolute location of lanthanide energy levels and the performance of phosphors. Journal of Luminescence 2006,122-123, 315-317.
- D. Dutczak, T. Justel, C. Ronda and A. Meijerink, Eu2+ Luminescence in Strontium Aluminates, JCCP, Royal Society of Chemistry, 2015, 15236-15249.