Absorbance
In very general terms, Absorption Spectroscopy is the spectroscopic technique that considers what happens when electromagnetic radiation of any wavelength passes through a material and some of these wavelengths are absorbed by the substance under study. Very often, visible light is used to analyse the material and the most common substances examined are solutions containing a dissolved sample.
The technique is widely used in analytical chemistry, medical & research labs to determine the concentration of an absorbing species in an (often unknown) solution and to quantify the amount in the sample. What is usually measured is the quantity of light absorbed by the test solution and expressed in units of Absorbance (A). For historical reasons, it absorbance was also called the Optical Density (OD).
The absorbance is related to the Transmittance (T) of a solution by the Absorbance Equation:
A = Log10(I0/I)
where I0 is the intensity of the light incident on the sample and I is the intensity of the light after passing through the sample.
So the Transmittance T is equal to I/I0 and the percent transmittance is simply %T = 100 (T)
When we want to calculate absorbance A from % transmittance we can use the relation
A = – log10(T)
Since there is a logarithmic relationship between absorbance and transmittance, some handy values to remember are 100%T = 0A, 10%T = 1.0A, 1%T = 2.0A, 0.1%T = 3.0A, and so on. In theory, zero % transmittance would correspond to infinite absorbance; no light gets through the sample and everything is absorbed.
Analytical labs are mostly interested in two things: identifying the chemical components in an unknown sample, and measuring how much of each component is present. The most common relationship used is the Beer-Lambert equation:
A = ε c l,
where ε is the molar extinction coefficient (also called the molar absorptivity or molar attenuation coefficient), c is the concentration of the species in the test solution in moles per litre, and l is the path length in cm. Usually measurements are carried out in 1 cm glass or quartz cuvettes, so the path length l = 1 cm. Most often it is the concentration c of an unknown species (the analyte) that we need to measure. This is achieved by making up a series of solutions of known concentration and constructing a calibration curve. From the slope of the curve we can calculate ε; since A is being experimentally measured, and we know l, we can then determine the value of c.
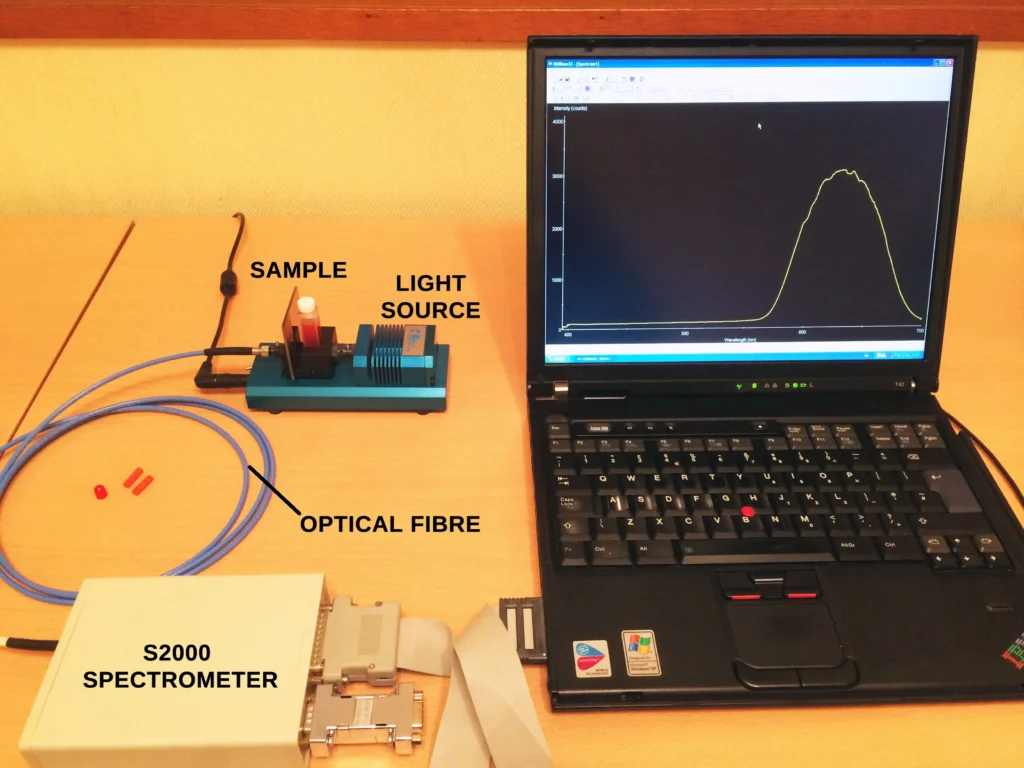
Measurements of spectral absorbance and transmittance can be considered the simplest experimental setups to perform in spectroscopy. Professional analytical labs measure these parameters frequently and the instruments are referred to as UV-Visible spectrophotometers. They are capable of handling multiple samples simultaneously with rapid data processing and high throughput for increased productivity. We can do this very simply at home too using modern miniature optical fibre spectrometers, which have been around for a few years, plus a few other components. Basically we need a source of broad-band visible white light (a tungsten filament lamp), a plastic, glass or quartz cuvette to hold the liquid sample, a couple of optical fibres and a small spectrometer & software. Here is a picture of my setup, using an Ocean Optics S2000 spectrometer, an integrated tungsten lamp & cuvette holder (also from Ocean Optics) and two optical fibres.

