Introduction
The above image is a sample of high purity caesium chloride crystals (CsCl). They are in a sealed glass ampoule and were prepared under high vacuum. At first glance, these appear to be fairly uninteresting plain white crystals, very typical of so many other inorganic and organic salts. But when these crystals are excited in a high voltage electric field they undergo a stunning and captivating transformation!
Watch what happens in this short video when we place the crystals near to an electric field…
The electric field here is being created by a small Tesla coil which generates a low current, very high frequency, high AC voltage.
As the crystals approach the electric field, this white salt suddenly gives off a fascinating bluish-white glow and the crystals start to turn blue, many of them even changing to a dark blue colour. When the electric field is removed, the blue-white glow immediately disappears. But the crystals have all turned blue:
This blue colour persists for several hours before the crystals slowly return back to their original white state.
So what is going on here?
F Centres
This fascinating effect is due to the formation of what are called F Centres or Colour Centres. The name comes from the German Farbezentrum (“Farbe” = colour and “zentrum” = centre).
An F centre is a specific type of crystallographic defect found within a crystal lattice. The defect is an anionic vacancy in the lattice that then becomes occupied by one or more unpaired electrons. This is shown schematically for the case of sodium chloride NaCl (common table salt) in the following diagram, (courtesy of VladVD via Wikipedia Commons).
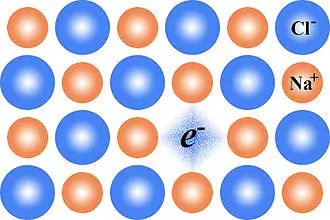
With the caesium chloride crystals in our demonstration above, electrons created by the high voltage have ejected chlorine atoms from the caesium chloride lattice and taken their places. Electrons that fill these lattice vacancies have a tendency to absorb light in the visible region of the spectrum. As a result, a normally transparent (or white) crystalline material undergoes this fascinating transformation and becomes coloured.
For the case of CsCl, the colour is blue. The intensity of the crystal’s colour is directly proportional to the number of F centres that are present in the lattice. Essentially, F centres represent a type of colour centre within the crystal structure.
F centres are common with many ionic compounds. They occur naturally with many metal oxides, such as zinc oxide, and can be induced by electric fields, as in this example, and also by photon excitation. The compounds most studied are the alkali metal halides.
More information on F centres can be found online at the usual sources so I will not elaborate here.
The high vacuum in the sample allows us to examine spectroscopically the bluish-white glow that appears above the crystals seen in the video. If we were to break open the glass ampoule, the pungent smell of chlorine gas would be detected!
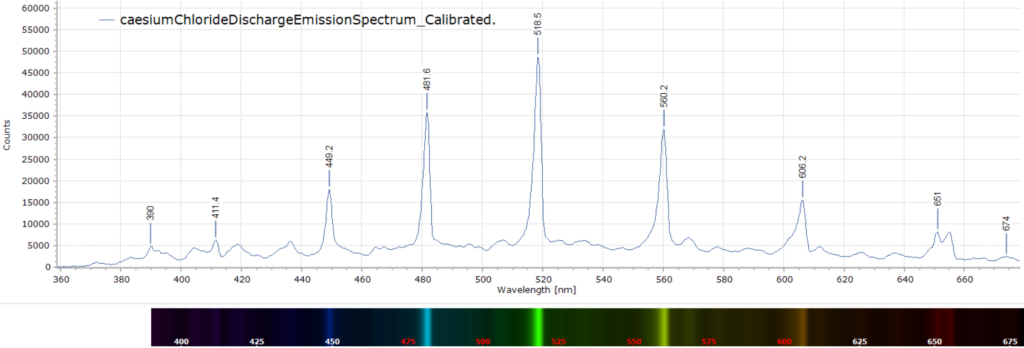
The spectrum of this glow discharge can be seen below recorded, as with many spectra in this blog, with an MS125 1/8 metre focal length spectrometer and linear CCD detector plus a fibre-optic feed.
I have to admit that I am having difficulty assigning the above emission lines to specific chemical species. The relevant atomic and molecular species involved in such a discharge must be Cs, Cs+, Cl2 and Cl–. The wavelength accuracy in the spectrum is +/- 1 nm.
So if anyone is able to explain the nature of these emission bands, please send me your comments and we can have a great chat.
Thanks!