Introduction
Does anyone remember having glow-in-the-dark stars, planets and constellation stickers that as kids we would stick to our bedroom walls and ceiling? I don’t think I am the only one to remember and enjoy this daily night time event. Once the bedroom light was switched off these stickers would glow green for a while, usually lasting several minutes before the glow eventually disappeared. It was a useful night-light for our parents after a scary bedtime story from Mum or Dad!
This slowly decaying glow is due of course to the chemical process of phosphorescence.
Essentially, phosphorescence is the emission of light from molecules in a very specific electronically excited energy state. You can check out this post for a more technical explanation of the different processes involved. As the molecules discard excess energy and return to their lowest energy state (called the ground state), this energy is emitted as photons of wavelengths longer than the original excitation light. The energy difference determines how much phosphorescence energy, in terms of its frequency, or wavelength, has been produced.
A more technical description of phosphorescence can be found here that explains how it differs from fluorescence and other energy deactivation processes in molecules.
As usual in these blogs we try to minimize the mathematics as much as possible.
The type of phosphorescent stickers I remember as a young boy were made of paper or thin card. You peeled off the silicone backing paper and then stuck them to your bedroom wall and ceiling. The actual phosphorescent material used in those days was likely to have been copper-doped zinc sulphide and it was reasonably effective as a phosphor. However, the glow properties did not last all that long after the excitation source (the bedroom light) was switched off.
Today, more efficient phosphors have become available that are based on strontium aluminate that has been doped with europium (Eu2+) and dysprosium (Dy3+) ions. A dopant is a kind of impurity element deliberately introduced in trace quantities into the host material’s crystal lattice in order to alter its electrical and/or optical properties.
Strontium aluminate is a white to pale yellow crystalline powder with the formula SrAl2O4 and its doped version has the general structural formula Eu:SrAl2O4. The actual phosphorescence originates from the emission of light from the Eu2+ ions. For a more detailed description and some experiments with strontium aluminate powders, you can read more in this post.
These glow-in-the dark materials are initially manufactured as pure powders, together with the added dopant ions. They can be obtained online at little cost from the usual sources in powder form. More typically, though, they are formulated with a non-reactive acrylic or epoxy resin in moulds to make the plastic stickers, small toys and glow-sticks that we see for sale in toy shops and elsewhere. These items are still extremely popular for decorating children’s bedrooms, as a quick look on Amazon will tell you. They are also very useful today as “persistent luminescent materials” for use in glow sticks with the emergency and rescue services, for escape paths in aircraft, for glow-in-the-dark collars for domestic pets, and many other applications.
Interestingly these products are also available in the form of phosphorescent paints for decorating various surfaces such as ornaments, pottery and personal decorative items, even your finger nails and face paint! The paints will glow in the dark after being irradiated with natural daylight, room light or long wavelength UV for a few minutes.
It is these phosphorescent paints that we will be examining in this post.
Objectives
We decided to evaluate the phosphorescent properties of these paints as much as possible in a home lab setting. It is assumed that the phosphor in the paints is Eu/Dy-doped strontium aluminate. The older type, Cu-doped zinc sulphide, is moisture sensitive which affects and degrades the phosphorescence properties. Since these paints are water-based acrylics, it is a safe assumption that we are dealing with doped strontium aluminate. The main objective is to record the spectral emission characteristics for each paint, which will change and be dependent on the coloured dyes in the paints.
An additional experiment, if it turned out to be useful, was to determine if we could measure the rate of decay of phosphorescent intensity from a suitable paint sample when the excitation light source has been switched off.
Materials and Methods
The paints were purchased for a few euros from one of the usual online sources (we all know which one) and came in a pack of 12 different colours.

There was no information on the box regarding the actual chemical composition of these paints, but all samples appeared to be thick acrylic based water soluble paints having been formulated with different coloured dyes or pigments and presumably with added strontium aluminate plus dopants to create the glow-in-the-dark effect as the manufacturer claimed on the box.
Experimental Setup
In an initial examination, several drops of each paint sample were spread onto black card and left to dry for 24 hours. Images of the samples, under normal room light conditions and also under long wavelength UV light can be seen here:
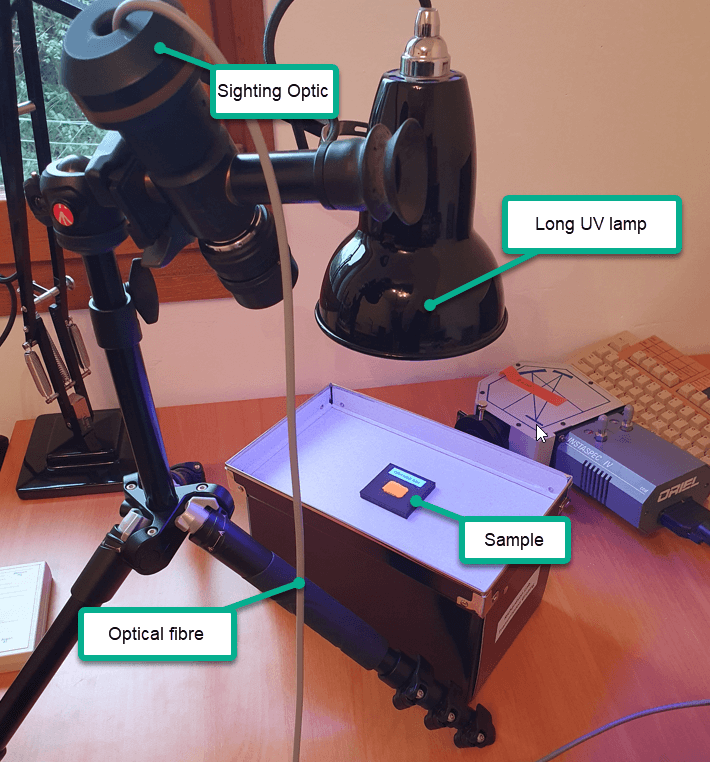
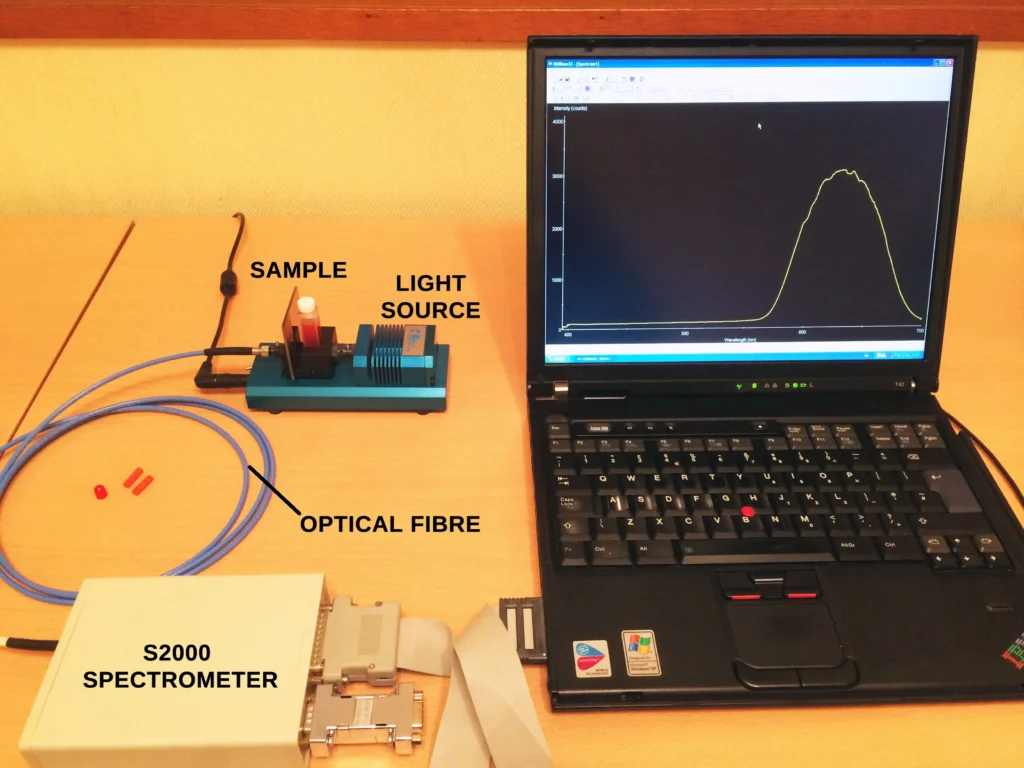
An Oriel Sighting Optic with a 50mm camera lens was focused onto a 2mm diameter spot for each dried paint sample. More details of this sighting optic device and its advantages can be found in this post. The sighting optic was connected to an Oriel MS125 spectrometer and CCD detector with a 100µm optical fibre and an emission spectrum recorded for each sample.
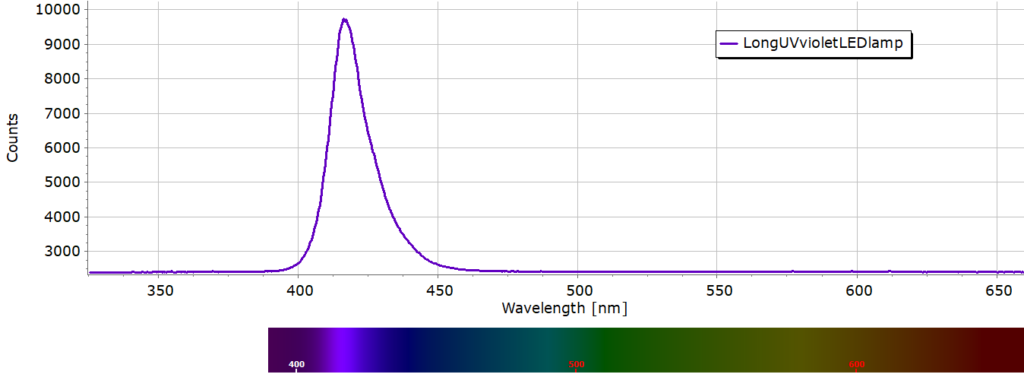
Ordinary room light was sufficient to produce some limited degree of luminescence but a long wavelength UV lamp provides increased emission intensity. For irradiating each paint sample we used a low cost high intensity violet coloured LED bulb shown in Fig. 3, screwed into an angle poise lamp. The LED’s have a peak emission around 415 nm as shown in Fig. 4.

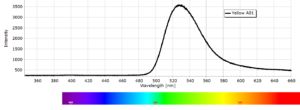
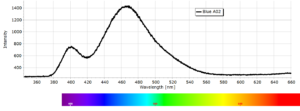
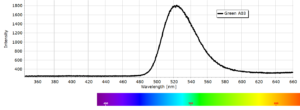
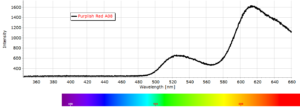
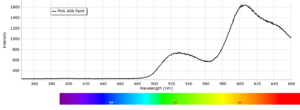
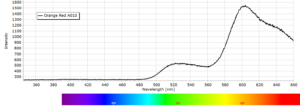
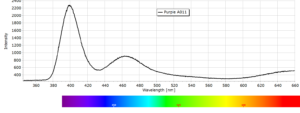
Phosphorescence Spectra
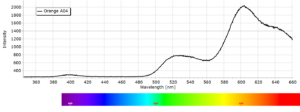
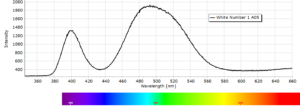
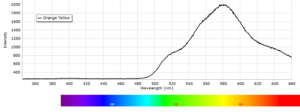
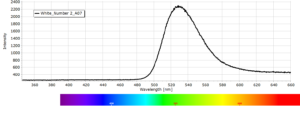
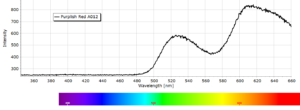
Phosphorescence spectra expressed as light intensity (arbitrary units) as a function of wavelength in nm for each paint sample are reported in the following image gallery.
Results and Discussion
To isolate small quantities of strontium aluminate, two of the more brightly phosphorescent paints were evaluated further.
Eu-doped strontium aluminate is described in the literature as being “slightly soluble in water”. Dispersed in an acrylic polymer may well modify its solubility properties but this was not investigated here.
Two samples of the most strongly luminescing paints were diluted with 25ml of a 1:1 water:iso-propanol mixture, shaken well, filtered and the filtrate left to settle overnight. The resultant samples were again “washed” with solvent to remove any remaining polymer. A typical image from one sample of the final, dried powder residue is shown here under both room light and UV light:


This 1 min video here shows the decay of phosphorescence from one of the paint samples.
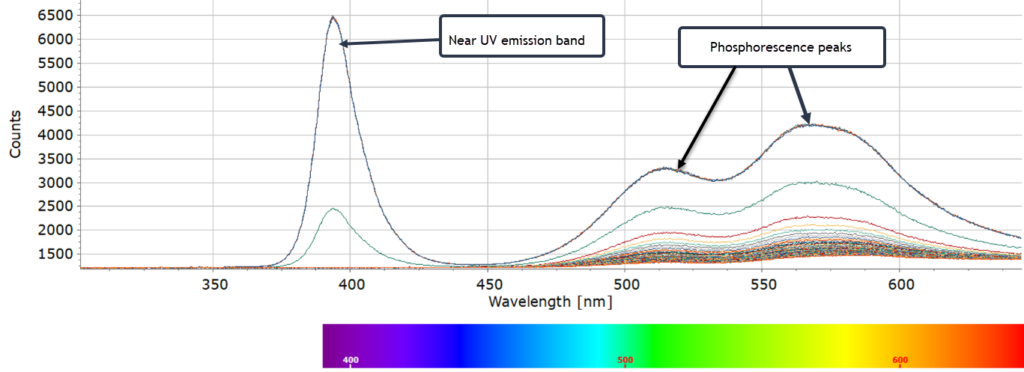
Rod cells in the human eye are very efficient at detecting very small changes in light intensity, particularly in the green and yellow regions where our rod cells are most sensitive. So although in the video we still see the phosphorescent glow from the sample, the actual photon intensity is very quickly reduced on a timescale of seconds as the spectra below show. The afterglow, nevertheless, persists for several minutes.
Conclusions
Phosphorescent spectra of a series of twelve luminescent paint samples have been produced showing the changes in wavelength (colour) as a function of dye or pigment used in each formulation.