
Introduction
The chemical element Selenium. Atomic number 34. Chemical symbol Se.
The quirky title I chose for this post – an element that can’t make up its mind – refers to the fact that selenium is generally considered a nonmetal. However, it has also been classed, by many investigators in the past, as a metalloid. A metalloid is an element whose physical and chemical properties are a blend of those of metals and nonmetals. So selenium, to some extent, falls between a metal and a nonmetal.
Selenium is in Group 16 of the Periodic Table (previously called Group VI A in the old naming system) and is situated between sulphur and tellurium within the group. Group 16 is also known as the Chalcogens or the ‘oxygen family’. The complete group, in increasing atomic number, consists of Oxygen, Sulphur, Selenium, Tellurium and the radioactive elements Polonium and Livermorium.
Selenium was discovered by the Swedish chemist Jöns Berzelius and his colleague Johan Gahn in 1817. Being the discoverers, they had the right to name the new element. Because tellurium (from the Latin tellus, meaning “Earth”) had been discovered a couple of decades previously and named in honour of our home planet, Berzelius and Gahn rewarded the Earth’s natural satellite by naming the new element selenium (after the Greek selène, meaning “Moon”).
Allotropy and Polymorphism
Falling between sulphur (definitely a nonmetal) and tellurium (a metalloid) selenium possesses physical and chemical properties of both its Group 16 neighbours. An important common feature of all the Group 16 elements is allotropy and polymorphism.
An allotrope is the property of an element to exist in two or more different forms. The classic example is carbon. Carbon has been known for many years to exist both as diamond and also graphite. More recently two additional allotropes of carbon have been added – graphene and the fullerenes.
The terms allotrope and allotropy are normally reserved for pure elements. When we refer to chemical compounds, the terms polymorph and polymorphism are normally employed, which refer to any solid material that is capable of existing in more than one form or crystal structure.
Selenium’s lighter neighbour sulphur, for example, occurs in more natural allotropes and polymorphs than any other element in the Periodic Table! I expect you only have to look at some of the NASA photos of the Jovian moon Io, where erupting sulphur volcanoes on this closest moon to Jupiter, are everywhere. It makes Io look like some giant planetary pizza!

The different allotropes of selenium can be interconverted by changing the temperature. The three principal allotropic forms are red selenium, grey selenium and black selenium. But then things get more complicated…
The red form of selenium is further sub-divided into 3 sub-types called α, β and γ. In addition there are also two synthetic allotropes called cyclo-Se6 and cyclo-Se7 plus an amorphous form, making 6 different forms in total. And that is just for the red form of selenium! These red forms do not conduct electricity, making red allotropic selenium a nonmetal, or at best a metalloid.
The image at the top of this post, for example, shows my sample of Se with all three forms apparently present, since the sample has been alternately heated and cooled. More about that later.
Structurally, natural red selenium consists of Se8 rings. It is the differences in the layering and packing of the rings that gives rise to the α, β and γ polymorphic forms.
At room temperature the most stable allotrope is grey selenium, which is crystalline and composed of unbranched helical chains. Grey selenium is a photoconductor and can conduct electricity, unlike the other two allotropes.
Commercially, the most common form is black selenium. It is amorphous and somewhat glassy in appearance, without any clear crystal structure, although it is thought to consist of rings of different sizes polymerized into helical chains. These have a very complex structure that can contain up to 1,000 atoms!
Biology
Physiologically, selenium is considered an essential trace element for humans. It is a cofactor in human biology and works with iodine to maintain correct thyroid gland function. But as with many other essential elements, there is only a very narrow range of concentration between what is considered the minimum daily requirement, for good health, and what becomes toxicity. In doses above 400 μg per day, selenium is considered toxic.
Applications
As far as applications go, these are relatively limited. Selenium-based light meters, using the photoelectric properties of the element, were very popular with photographers for many years in the days of silver halide photography. A typical example of a selenium based light meter is shown here:

Se continues to be used in electronics for its photoconductive properties, in solar cells and also in photocopier toners and DC surge protectors. The largest commercial use of Se is in glass manufacturing. This is because the red colour of selenium compounds can cancel out the yellow tint of molten glass formulations that are a constant problem during glassmaking and which are due to iron impurities.
Sample Preparation
In keeping with the main spectroscopy theme of this site, the nature of the emission spectrum of selenium vapour produced in an electrical discharge has been recorded.
As we shall see below, the spectral features differ very significantly from the narrow atomic line spectra seen for elements such as mercury (Hg), neon (Ne) and the other noble gases reported elsewhere on this site.
A sample of pure elemental selenium was obtained from the company SmartElements based in Vienna, Austria. The sample was claimed to have a purity of 99.99%. It was supplied in a sealed 12mm diameter glass tube, making it suitable for producing a high voltage a.c. discharge. An image of the sample is shown here:
In order to get enough selenium in the gas phase, the sample needs to be heated. This can be done by immersing the tube in boiling water for a few minutes and then connecting it to an electrical circuit to generate the discharge.
However, I found the most effective way is to use a hot air gun which will quickly heat the sample to about 150-200°C. The tube is already set up and connected to a high frequency a.c. discharge and all you have to do is switch on the power. After a few seconds of heating with the gun, a rather beautiful electric blue glow appears in the tube, which gradually brightens. This discharge can be maintained by periodically applying the heat gun.
A short video showing this is provided here:
If you watched the video just above, you should have seen the black, grey and red allotropic forms of pure selenium, slowly evaporate when heated. This increases the vapour pressure of selenium in the tube and increases the blue glow produced. This glow is due to atomic Se and Sen molecular emission lines, resulting in complex spectral features and peaks.
Emission Spectra
From what we said earlier about the different polymorphs and molecular forms of selenium, it should come as no surprise that the spectrum is complex for a pure element, to say the least.
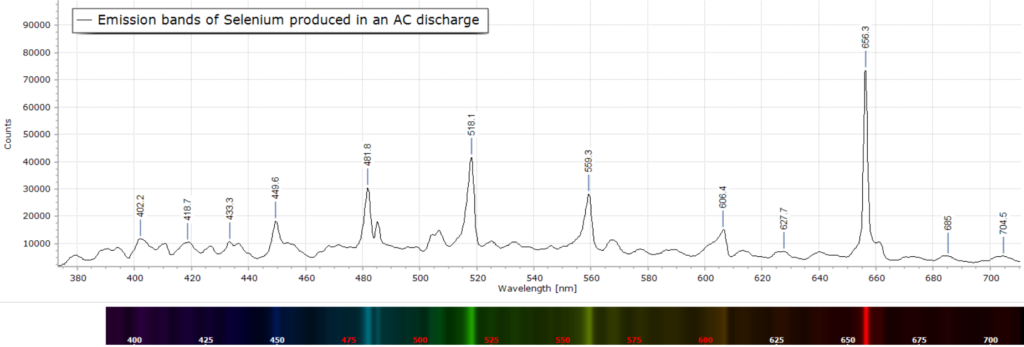
The low resolution spectrum of selenium in the glow discharge is shown in fig. 1 across the visible. This spectrum was obtained with a CCTV camera lens attached to a sighting optic and optical fibre, MS125 spectrometer and CCD detector. This spectrum consists of a series of largely unresolved emission bands and peaks.
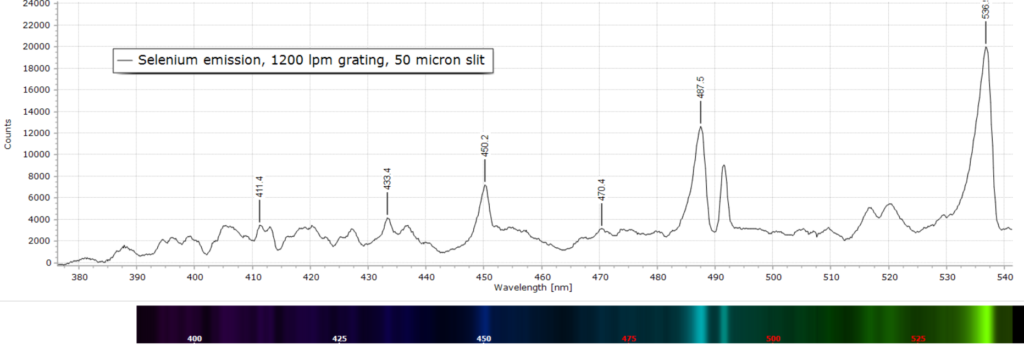
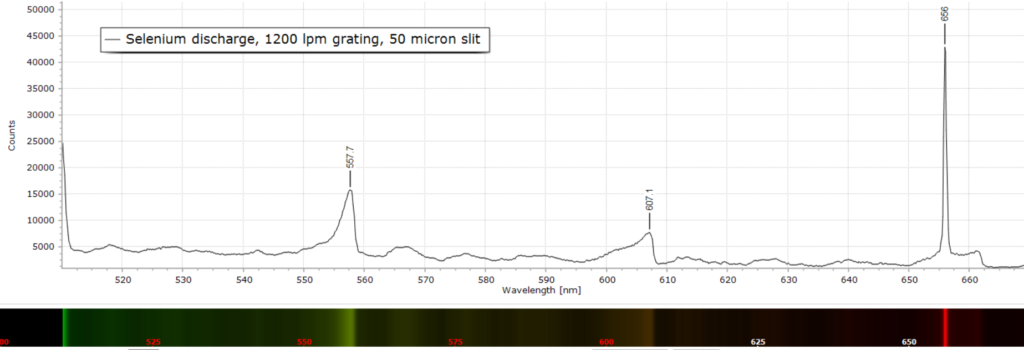
At slighter higher resolution with a 1200 lpm grating, the overall picture remains the same in Figs 2 through 4.
Spectral peak assignment is not possible in this case, apart from labelling the wavelength maxima.
Conclusions
I began this post by stating in the introduction that the commercial applications of selenium are somewhat limited. However, this rather unusual element may nonetheless have a brighter future:
Thin-film deposits of Se, produced from the vapour phase, are now showing promise as ultra fast radiation detectors in medical imaging. PET (positron emission tomography) is a good example. Also, high-energy physics applications for detecting Cerenkov radiation, in optical communications and in time-domain spectroscopy have all began to produce some interesting results with vapour-deposited selenium.
So let’s not yet relegate selenium to one of the elements with little or no use here on this precious planet Earth of ours. All the elements, beyond hydrogen and helium, originally formed from supernova explosions in our universe’s distant past. Each are fascinating in their own ways and worthy of continued, detailed study.


Very cool 🙂
Be careful you don’t accidentally break that tube!