Introduction
Spectrum tubes are a very useful way to introduce science students to the study of the interaction of light photons with matter and the subject of optical spectroscopy. They are often seen in demonstrations in high schools and early university lab classes to teach the basic principles of quantum theory and atomic and molecular transitions.
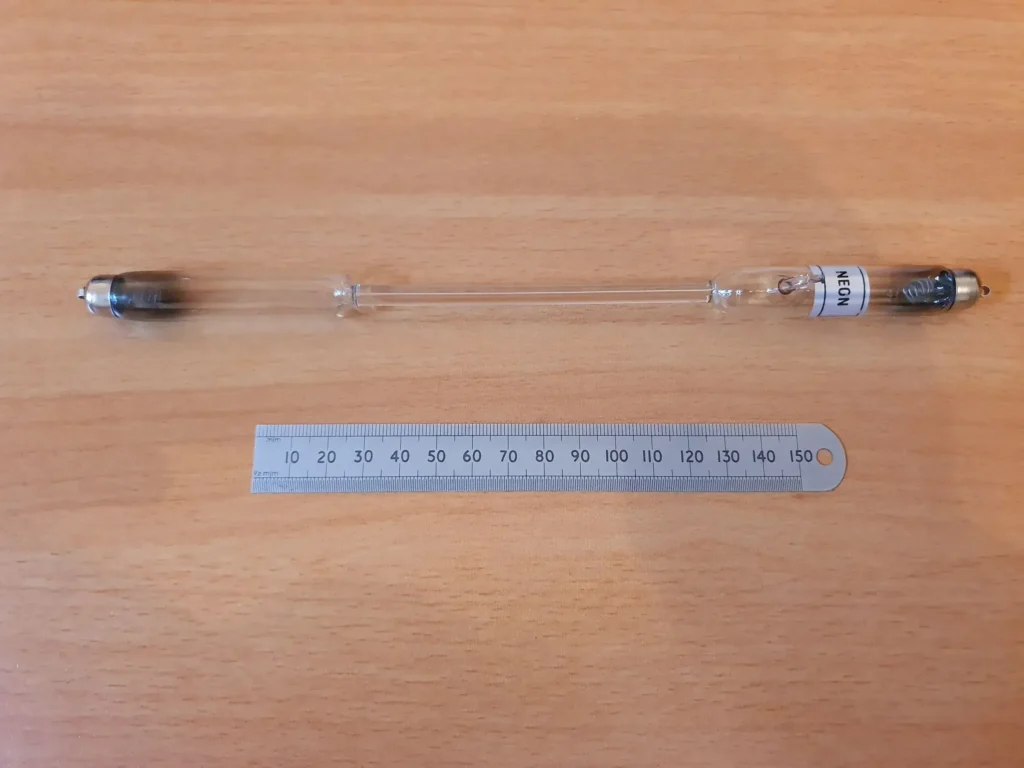
A typical tube, such as the one shown in the image above, usually contains a gas, such as hydrogen, neon, mercury vapour, etc. at low pressure, that will produce narrow atomic emission lines when an electric current is passed through it. The glass tubes are prepared under vacuum and a small quantity of the sample gas, whose spectrum is to be studied, is bled into the tube to a pressure of about 5-20 torr.
Typically, the tubes are 20-30 cm long with a middle “capillary” section for observing the spectrum. Metal contacts are attached at each end of the tube during manufacture and the tube is inserted into a power supply between spring-loaded electrodes. The following two pictures depict the high voltage power supply that is needed to produce the discharge.
The voltage needed to generate the discharge in these tubes is typically around 1000-1500V with a current of about 10mA. This high voltage, the value of which depends on the actual gas pressure in the tube, will then exceed the breakdown voltage of the gas, current then flows and completes the electrical circuit. Thus the tube lights up and produces atomic or molecular emission lines and bands characteristic of the atomic or molecular species under study.
A Rainbow of Colours
- colours of spectrum tubes
Experimental Setup
The spectra in the next section were all obtained by placing one end of a 600 μm optical fibre close to each emission tube with the other end, via a small collimator lens, focused onto the entrance slit (25μm) of an Oriel-Newport MS125 spectrometer. Spectra were recorded with either a 600 lpm or 1200 lpm grating.
Depending on tube intensity, data acquisition times varied from 50 to 1500 accumulations of 1 millisecond exposures with an Andor iDus 420 CCD detector thermoelectrically cooled to -60C below ambient. Similar compact optical fibre spectrometers and configuration settings can produce similar resluts.
All spectra were background corrected.




















What purpose does the low pressure of the gas serve in these tubes?
Hi,
Several reasons. The pressures are relatively low to allow the applied voltages across the electrodes of the tube to be fairly low, typically 1000 to 1500 Volt which is “low’ for this application. This voltage then ionizes the atoms/molecules of the gas to form ions, excited atomic and molecular species, and allows a current to flow. The excited atomic or molecular species are produced from collisions with electrons and atoms, but then release their excess energy in the form of light at wavelengths that are characteristic of the gas.
A second reason for low pressures is that the emission lines will be narrow and not subject to pressure broadening. Various mechanisms that broaden spectral lines are covered in this blog post https://stevesopenlab.fr/emission-spectra-of-the-noble-gases/ This is important when the line emission wavelengths are used to calibrate spectrometers, such a spectrum tubes for Mercury and the noble gases Neon, Krypton, etc.
Possibly a minor(economic) advantage is that this reduces the cost per tube by the manufacturer since they are using less gas. but that is a very minor reason.
Hope this helps.
Any questions, feel free.
Thanks for the comment,
Steve