The Beer-Lambert Law and Spectrophotometric Analysis
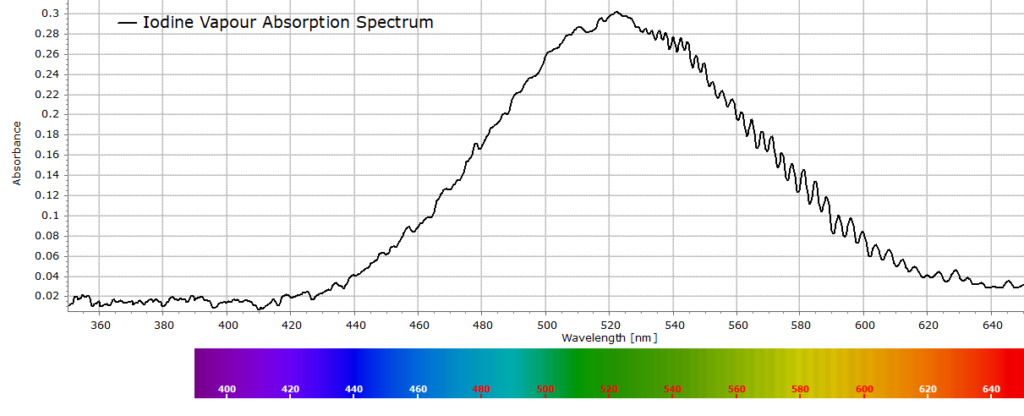
6 Minute ReadIntroduction One of the very first posts on this site introduced the basics of Absorption Spectroscopy and the concepts of Absorbance and Transmittance of light through homogeneous materials. In this article we are going to consider this topic in a little more detail through the Beer-Lambert law and use the relationship to determine the concentration […]
The Beer-Lambert Law and Spectrophotometric Analysis Read More »