Introduction
Measuring the fluorescence from highlighter pens is always an interesting educational project to try. This experiment has become very popular in schools and colleges in science classes to demonstrate the basic principles of fluorescence and spectra. Very often green, blue or violet laser pointers are used to excite the sample. This is the lowest cost option, but any broad band UV source can be tried with the appropriate safety precautions.
There are a couple of ways this can be done:
1) The simplest and most basic method is to use a small white card and cover it with highlighter ink to create a sort of colour swatch. Several layers of ink coatings may be necessary to increase the colour density of the dye. Leaving a layer to dry for a few minutes between coats will give the best results.
A laser pointer is then directed at the card which excites fluorescence from the highlighter dye molecules adsorbed on the card. The spectrum can be captured with a cheap homemade spectrometer (there are even cardboard varieties to be found online). The spectrum is then captured with a video or phone camera and a screen shot of the whole spectrum can be taken. This raw spectrum can then be converted by software to obtain the spectral line profile.
This is the “quick and dirty” method that has produced some useful qualitative results in a classroom setting.


2) A more sophisticated and accurate method, which can still be done in the classroom or in a home lab with the right equipment, is to dissolve some of the highlighter ink in a suitable solvent. This is then added to a spectrophotometric cuvette and a small spectrometer with optical fibre connections is used to record the fluorescence spectrum. This is a very reliable and reproducible the method and is the one that we describe below.
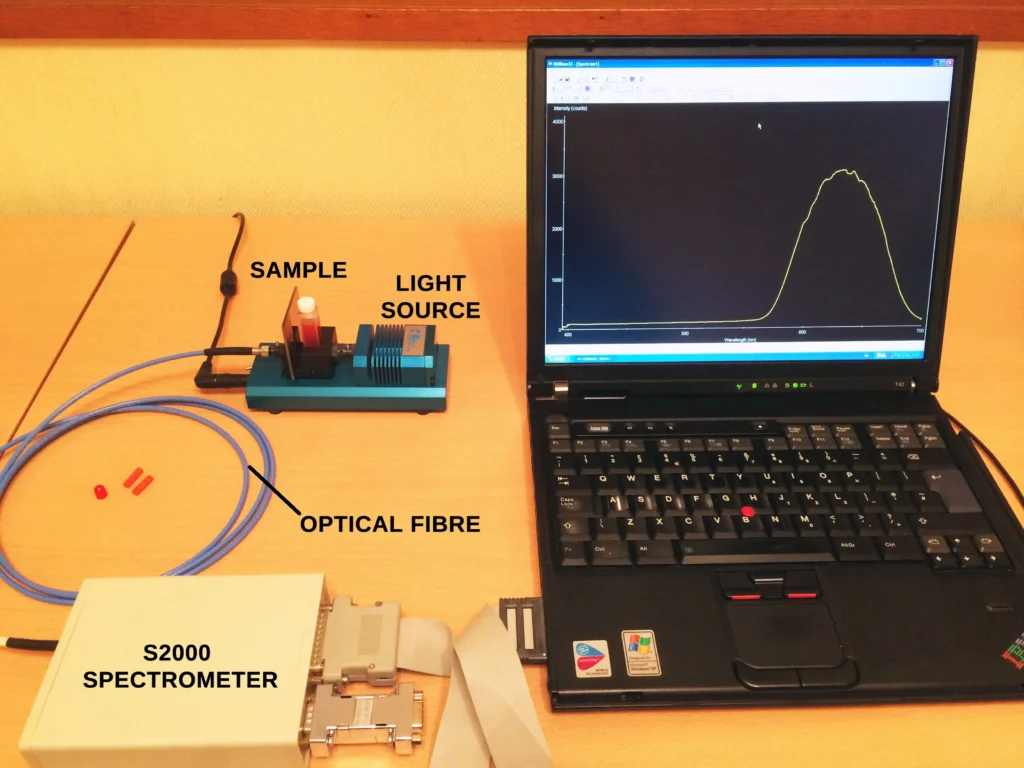
The Experimental Setup
First of all, the fluorescent dye must be extracted from the highlighter pen. To achieve this each pen was dipped in a 1:1 mixture of water and iso-propanol (propan-2-ol) for a few seconds. Pure tap water works just as well but the alcohol helps extraction of the dye, especially if the highlighter pens are old and the nibs are starting to dry. The extracted dye solutions were then transferred to 1 cm path length quartz spectrophotometric cuvettes.
The following 3 images show the initial setup to check feasibility.


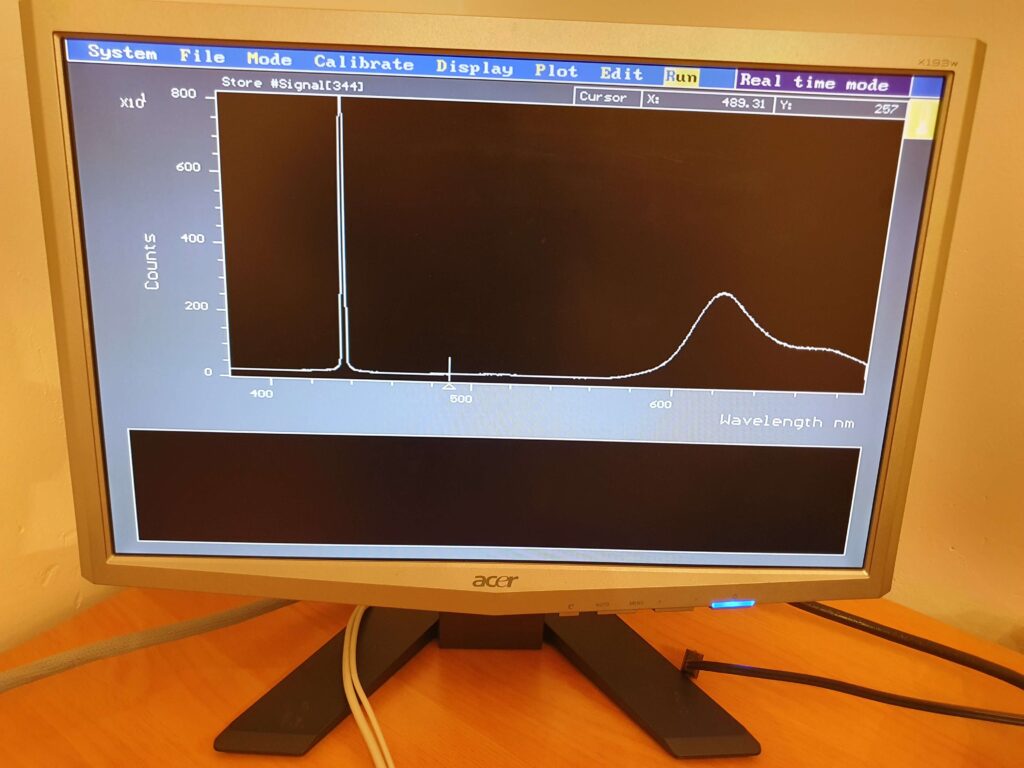
Fluorescence Spectra
Fluorescence spectra of dyes extracted from highlighter pens can be seen in the figures below. The initial test above used an Oriel MS125 spectrometer in combination with a sensitive CCD detector. The results below present the fluorescence spectra recorded with an Ocean Optics S200 fibre-optic spectrometer and associated software, and then imported into Spectragryph© free spectroscopy software. Spectragryph is an excellent spectrum data acquisition and processing application written by Dr. Friedrich Menges that is a free download for all non-commercial users.
A Few Words on the Chemistry
Dyes used in highlighter pens come from several different organic dye families with different molecular structures. Some pen colours have been developed by mixing one dye with another. Below are some of the main examples…
Yellow highlighter pens usually consist of pyranine which is a pyrene-based dye, although Fluorescein is also be found in some types of yellow pens. Pyranine is also known as Solvent Green 7.



Blue ink pens are usually based on the triphenylmethane (TPM) dye structure. A common dye used is Acid Blue 9. TPM dyes are only weakly fluorescent, if at all – they have a low fluorescence quantum yield. This is largely because of more efficient non-radiative deactivation processes described more completely in this post on the theory of fluorescence. This is reason why there is little or no fluorescence from the blue highlighter in the earlier image.

Pink highlighters often used a Rhodamine dye such as Rhodamine B (also known as Basic Violet 10 or Brilliant Pink B). If a more purple coloured ink is required Rhodamine B can be combined with a TPM dye.